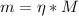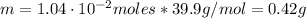Chemistry, 31.03.2020 04:40 Gogontle8347
A chemist must prepare 800.0mL of sodium hydroxide solution with a pH of 12.10 at 25°C. She will do this in three steps: Fill a 800.0mL volumetric flask about halfway with distilled water. Weigh out a small amount of solid sodium hydroxide and add it to the flask. Fill the flask to the mark with distilled water. Calculate the mass of sodium hydroxide that the chemist must weigh out in the second step. Round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2
You know the right answer?
A chemist must prepare 800.0mL of sodium hydroxide solution with a pH of 12.10 at 25°C. She will do...
Questions

Mathematics, 02.12.2020 02:50



English, 02.12.2020 02:50

Mathematics, 02.12.2020 02:50


Mathematics, 02.12.2020 02:50



Mathematics, 02.12.2020 02:50

Mathematics, 02.12.2020 02:50



Mathematics, 02.12.2020 02:50



English, 02.12.2020 02:50



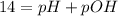
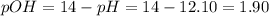
![pOH = -log ([OH^{-}])](/tpl/images/0572/8536/5638e.png)
![[OH]^{-} = 10^{-pOH} = 10^{-1.90} = 0.013 M](/tpl/images/0572/8536/7c501.png)
![\eta = ([OH]^{-})*V = 0.013 mol/L * 0.800 L = 1.04 \cdot 10^{-2} moles](/tpl/images/0572/8536/c1790.png)