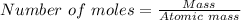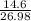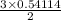Chemistry, 31.03.2020 04:37 chantianabess36
Sulfuric acid dissolves aluminum metal according to the following reaction: 2Al(s)+3H2SO4(aq)→Al2(SO4)3(aq)+3H2 (g)2Al(s)+3H2SO4(aq)→Al2(SO4)3(aq)+ 3H2(g) Suppose you wanted to dissolve an aluminum block with a mass of 14.6 gg . Part A What minimum mass of H2SO4H2SO4 would you need? Express your answer in grams.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
Sulfuric acid dissolves aluminum metal according to the following reaction: 2Al(s)+3H2SO4(aq)→Al2(SO...
Questions



Social Studies, 01.01.2021 18:40


SAT, 01.01.2021 18:40


Mathematics, 01.01.2021 18:40




History, 01.01.2021 18:50


Geography, 01.01.2021 18:50


History, 01.01.2021 18:50

Mathematics, 01.01.2021 18:50


Arts, 01.01.2021 18:50

Social Studies, 01.01.2021 18:50

Social Studies, 01.01.2021 18:50