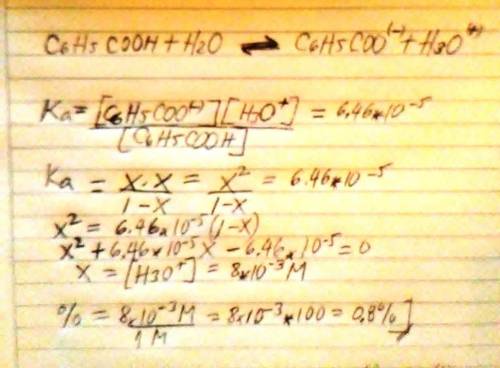The acid ionization constant for benzoic acid (C6H5COOH) is 6.46 × 10−5 . Compare the percent ionization of 1.00 M benzoic acid in water with its percent ionization in 0.500 M sodium benzoate solution. Support your comparison with calculations similar to those in Model 1.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3
You know the right answer?
The acid ionization constant for benzoic acid (C6H5COOH) is 6.46 × 10−5 . Compare the percent ioniza...
Questions


Computers and Technology, 03.01.2020 20:31

Business, 03.01.2020 20:31

Social Studies, 03.01.2020 20:31

Health, 03.01.2020 20:31



History, 03.01.2020 20:31


English, 03.01.2020 20:31




Biology, 03.01.2020 20:31

Mathematics, 03.01.2020 20:31

Social Studies, 03.01.2020 20:31




History, 03.01.2020 20:31