

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3

Chemistry, 23.06.2019 05:00
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
The natural distribution of the isotopes of a hypothetical element is 60.795% at a mass of 281.99481...
Questions

History, 05.11.2019 10:31


History, 05.11.2019 10:31




Biology, 05.11.2019 10:31



Chemistry, 05.11.2019 10:31

Biology, 05.11.2019 10:31


English, 05.11.2019 10:31


Mathematics, 05.11.2019 10:31

Mathematics, 05.11.2019 10:31


Advanced Placement (AP), 05.11.2019 10:31


History, 05.11.2019 10:31

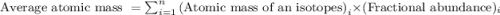 .....(1)
.....(1)![\text{Average atomic mass of element}=[(281.99481\times 0.60795)+(283.99570\times 0.22122)+(286.99423\times 0.17083)]\\\\\text{Average atomic mass of element}=283.291amu](/tpl/images/0572/8936/1f7da.png)


