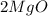Chemistry, 31.03.2020 14:41 teesoprettyy
12g of magnesium ribbon was heated in a crucible. At the end of the reaction 20g of magnesium oxide had been produced. What mass of oxygen had reacted with the magnesium?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1
You know the right answer?
12g of magnesium ribbon was heated in a crucible. At the end of the reaction 20g of magnesium oxide...
Questions


English, 16.12.2020 21:10

Mathematics, 16.12.2020 21:10

English, 16.12.2020 21:10

History, 16.12.2020 21:10



Mathematics, 16.12.2020 21:10

Mathematics, 16.12.2020 21:10

Social Studies, 16.12.2020 21:10





Health, 16.12.2020 21:10


History, 16.12.2020 21:10

Mathematics, 16.12.2020 21:10

Mathematics, 16.12.2020 21:10

Mathematics, 16.12.2020 21:10

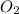 reacted with the magnesium
reacted with the magnesium