
Chemistry, 31.03.2020 14:40 ashley232323
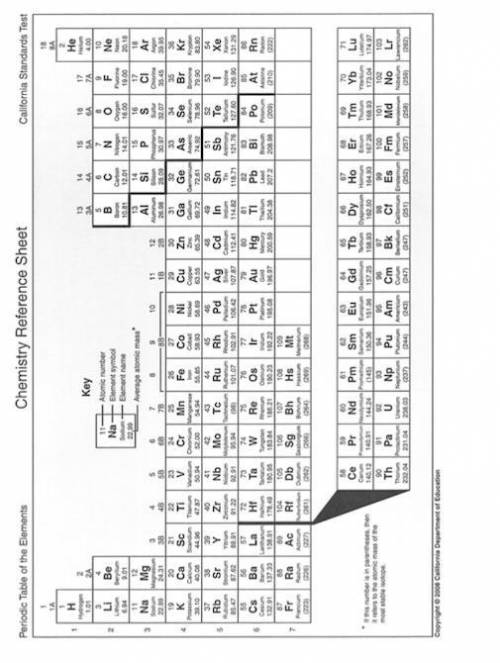
Solve the below problems relating to the molality of NaNO3 in aqueous solutions. Make use of the Periodic Table on page 2 to calculate the molar masses.
(i) A solution is prepared using 17 g of water and 1 g of NaNO3. What is the molality of the NaNO3 solution?
(ii) In order to prepare a solution of 2 molal NaNO3 using 30 g of water,
what mass of NaNO3 is required?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3

Chemistry, 23.06.2019 07:30
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes no correct anwser get brainliest
Answers: 1
You know the right answer?
Solve the below problems relating to the molality of NaNO3 in aqueous solutions. Make use of the Per...
Questions


Mathematics, 25.12.2020 02:40

Social Studies, 25.12.2020 02:40



Health, 25.12.2020 02:40

Mathematics, 25.12.2020 02:40

Mathematics, 25.12.2020 02:40

Mathematics, 25.12.2020 02:40

Physics, 25.12.2020 02:40

Mathematics, 25.12.2020 02:40



SAT, 25.12.2020 02:40

SAT, 25.12.2020 02:40



SAT, 25.12.2020 02:40


Engineering, 25.12.2020 02:40



