2. If I have 45 g of Carbon, to find the number of moles of carbon I would *
divide by molar m...

Chemistry, 31.03.2020 16:16 heavendl13
2. If I have 45 g of Carbon, to find the number of moles of carbon I would *
divide by molar mass (12.01 g)
multiply by molar mass (12.01 g)
divide by Avogadro's number (6.02 x 10^23)
multiply by Avogadro's number (6.02 x 10^23)
Sushmit

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
You know the right answer?
Questions




Mathematics, 09.10.2020 21:01

Mathematics, 09.10.2020 21:01


Mathematics, 09.10.2020 21:01

History, 09.10.2020 21:01

English, 09.10.2020 21:01




Social Studies, 09.10.2020 21:01

Mathematics, 09.10.2020 21:01



Arts, 09.10.2020 21:01

English, 09.10.2020 21:01

History, 09.10.2020 21:01

English, 09.10.2020 21:01

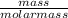
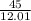 = 3.75moles
= 3.75moles 

