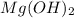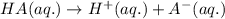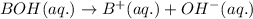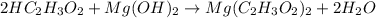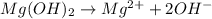2HC2H3O2 + Mg(OH)2 = Mg(C2H3O2)2 + 2H2O


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 17:50
The only bonds in a formula unit of caf2 are nonpolar covalent polar covalent ionic metallic
Answers: 1
You know the right answer?
Identify the base in this acid-base reaction:
2HC2H3O2 + Mg(OH)2 = Mg(C2H3O2)2 + 2H2O
2HC2H3O2 + Mg(OH)2 = Mg(C2H3O2)2 + 2H2O
Questions

Physics, 11.07.2019 14:30





Mathematics, 11.07.2019 14:30



Spanish, 11.07.2019 14:30





Chemistry, 11.07.2019 14:30

English, 11.07.2019 14:30


English, 11.07.2019 14:30

History, 11.07.2019 14:30


Chemistry, 11.07.2019 14:30