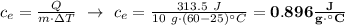Chemistry, 01.10.2019 19:50 justinhk10
Exactly 313.5 j will raise the temperature of 10.0 g of a metal from 25.0 c to 60.0 c. what is the specific heat of the metal?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 23.06.2019 09:20
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2

Chemistry, 23.06.2019 11:50
How many moles of an ideal gas would occupy a 25.0 liter container when the temperature is 295 k and the pressure is 0.850 atm?
Answers: 2
You know the right answer?
Exactly 313.5 j will raise the temperature of 10.0 g of a metal from 25.0 c to 60.0 c. what is the s...
Questions



Mathematics, 02.02.2021 18:30


Social Studies, 02.02.2021 18:30




Mathematics, 02.02.2021 18:30


English, 02.02.2021 18:30


Mathematics, 02.02.2021 18:30



Mathematics, 02.02.2021 18:30


Mathematics, 02.02.2021 18:30

Chemistry, 02.02.2021 18:30

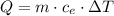 we can clear specific heat:
we can clear specific heat: