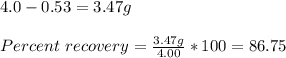Chemistry, 30.12.2019 17:31 SunsetPrincess
The solubility of acetanilide in hot water (5.5 g/100 ml at 100 c) is not very great, and it has some solubility in cold water (0.53 g/100 ml at 0 c). what would be the maximum theoretical percent recovery from the crystallization of 4.0 g of acetanilide from 100 ml of water (assuming the solution is chilled to 0 c for filtration). show your calculations.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
The solubility of acetanilide in hot water (5.5 g/100 ml at 100 c) is not very great, and it has som...
Questions



Mathematics, 05.10.2019 15:00


History, 05.10.2019 15:00

Social Studies, 05.10.2019 15:00

Biology, 05.10.2019 15:00




Spanish, 05.10.2019 15:00

Geography, 05.10.2019 15:00


Mathematics, 05.10.2019 15:00


Mathematics, 05.10.2019 15:00


Business, 05.10.2019 15:00


English, 05.10.2019 15:00