
Chemistry, 01.04.2020 05:41 ashtonbillups
At 25 oC, hydrogen iodide breaks down very slowly to hydrogen gas and iodine vapor with a rate constant of 2.4 x 10-21L/mol. s. If 0.0100 mol of HI(g) is placed into a 1.0 L container at 25 oC, how long will it take for the concentration of HI to reach 0.00900 mol/L?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:30
4nh3+5o2--> 4no+6h20what is the total number of moles of h2o produced when 12 mole of nh3 is completely consumed?
Answers: 3

Chemistry, 22.06.2019 03:40
Astudent is given a sample of a blue copper sulfate hydrate. he weighs the sample in a dry covered porcelain crucible and got a mass of 23.875 g for the crucible, lid, and sample. the mass of the empty crucible and lid was found earlier to be 22.652 g. he then heats the crucible to expel the water of hydration, keeping the crucible at red heat for 10 minutes with the lid slightly ajar. on colling, he finds the mass of crucible, lid, and contents to be 23.403 g. the sample was changed in the process to very light clue anhydrous cuso4. if there are again 100.0 g of hydrate, how many grams of cuso4 are in it? how many moles of cuso4? (hint: molar mass of cuso4 = 159.6 g / mole. what per cent of the hydrate is cuso4? you may convert the mass of cuso4 to moles.)
Answers: 3

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
You know the right answer?
At 25 oC, hydrogen iodide breaks down very slowly to hydrogen gas and iodine vapor with a rate const...
Questions


Mathematics, 01.07.2019 03:40


History, 01.07.2019 03:40



Social Studies, 01.07.2019 03:40


History, 01.07.2019 03:40

Mathematics, 01.07.2019 03:40




History, 01.07.2019 03:40


Chemistry, 01.07.2019 03:40

Mathematics, 01.07.2019 03:40

Mathematics, 01.07.2019 03:40

History, 01.07.2019 03:40

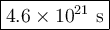
![\dfrac{1}{\text{[A]}} =\dfrac{1}{\text{[A]}_{0}}+ kt](/tpl/images/0575/0646/44bc3.png)
![\begin{array}{rcl}\dfrac{1}{\text{[A]}} & = & \dfrac{1}{\text{[A]}_{0}}+ kt\\\\\dfrac{1}{0.00900 }& = & \dfrac{1}{0.0100} + 2.4 \times 10^{-21} \, t\\\\111.1&=& 100.0 + 2.4 \times 10^{-21} \, t\\\\11.1& = & 2.4 \times 10^{-21} \, t\\t & = & \dfrac{11.1}{ 2.4 \times 10^{-21}}\\\\& = & \mathbf{4.6 \times 10^{21}}\textbf{ s}\\\end{array}\\\text{It will take $\large \boxed{\mathbf{4.6 \times 10^{21}}\textbf{ s}}$ for the HI to decompose}](/tpl/images/0575/0646/4ef33.png)



