
Chemistry, 01.04.2020 05:43 payshencec21
For the equilibrium
2IBr(g) ⇌ I₂(g) + Br₂(g) Kc = 8.50 × 10⁻³ at 150°C.
If 0.0600 mol of IBr is placed in a 1.0-L container, what is the partial pressure of I₂(g) in atm after equilibrium is reached?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1


Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
For the equilibrium
2IBr(g) ⇌ I₂(g) + Br₂(g) Kc = 8.50 × 10⁻³ at 150°C.
If 0...
2IBr(g) ⇌ I₂(g) + Br₂(g) Kc = 8.50 × 10⁻³ at 150°C.
If 0...
Questions

Social Studies, 02.07.2019 07:20








Mathematics, 02.07.2019 07:20





Computers and Technology, 02.07.2019 07:20

Computers and Technology, 02.07.2019 07:20

Computers and Technology, 02.07.2019 07:20

Social Studies, 02.07.2019 07:20



![[IBr]i = \frac{0.0600mol}{1.0L} = 0.060M](/tpl/images/0575/0702/18a8d.png)
![Kc = 8.50 \times 10^{-3} = \frac{[I_2][Br_2]}{[IBr]^{2} } = \frac{x^{2} }{(0.060-x)^{2} } \\\\x = 0.00507](/tpl/images/0575/0702/c2c7d.png)
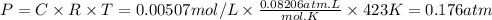
![\text{[IBr]} = \dfrac{\text{0.0600 mol}}{\text{1.0 L}} = \text{0.0600 mol/L}](/tpl/images/0575/0702/bae1e.png)
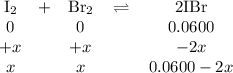
![\begin{array}{rcl}K_{\text{c}}&=&\dfrac{\text{[IBr]}^{2}} {\text{[I$_{2}$][Br]$_{2}$}}\\\\8.50 \times 10^{-2}&=&{\dfrac{(0.0600 - 2x)^{2}}{x^{2}}}& &\\\\0.2915x & = &{\dfrac{0.0600 - 2x}{x}}& &\\\\0.2915x & = &0.0600 - 2x\\\\2.2915x & = & 0.0600\\x & = & \textbf{0.026 18 mol/L}\\\end{array}\\](/tpl/images/0575/0702/36fc0.png)



