
Chemistry, 01.04.2020 13:36 genyjoannerubiera
Calculate the volume occupied by 272g
of methane at a pressure of 250 kPa and a
temperature of 54°C.
(R = 8.31JK-1 mol-1; M, methane = 16.0)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1


Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
Calculate the volume occupied by 272g
of methane at a pressure of 250 kPa and a
temperatur...
of methane at a pressure of 250 kPa and a
temperatur...
Questions

Physics, 07.10.2019 15:50




Business, 07.10.2019 15:50


Computers and Technology, 07.10.2019 15:50

History, 07.10.2019 15:50


Mathematics, 07.10.2019 15:50

Mathematics, 07.10.2019 15:50


Mathematics, 07.10.2019 15:50




Biology, 07.10.2019 15:50

Mathematics, 07.10.2019 15:50

English, 07.10.2019 15:50

Social Studies, 07.10.2019 15:50

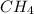 ) = 272 grams
) = 272 grams 

