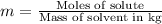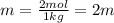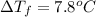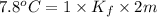Chemistry, 01.04.2020 18:36 kwarwick0915
Two moles of a nonelectrolyte solute are dissolved in 1 kg of an unknown solvent. the solution freezes at 7.8°c below its normal freezing point. what is the molal freezing-point constant of the unknown solvent? suggest a possible identity of the solvent.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1


Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 02:30
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2
You know the right answer?
Two moles of a nonelectrolyte solute are dissolved in 1 kg of an unknown solvent. the solution freez...
Questions

Mathematics, 17.02.2020 19:31

English, 17.02.2020 19:31










Mathematics, 17.02.2020 19:32


Mathematics, 17.02.2020 19:32


Biology, 17.02.2020 19:32




Mathematics, 17.02.2020 19:32

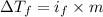
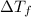 =depression in freezing point =
=depression in freezing point = 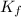 = freezing point constant
= freezing point constant