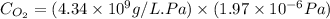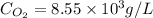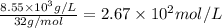Chemistry, 01.04.2020 18:52 20cschultz
Given the Henry’s law constant for O2(4.34*109Pa) at 25° C, calculate the molar concentration of oxygen in air-saturated and O2saturated water. (4 points)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1


You know the right answer?
Given the Henry’s law constant for O2(4.34*109Pa) at 25° C, calculate the molar concentration of oxy...
Questions

English, 18.02.2021 01:40

Mathematics, 18.02.2021 01:40

History, 18.02.2021 01:40


Mathematics, 18.02.2021 01:40


Arts, 18.02.2021 01:40

Computers and Technology, 18.02.2021 01:40





Mathematics, 18.02.2021 01:40


Mathematics, 18.02.2021 01:40

Spanish, 18.02.2021 01:40


Computers and Technology, 18.02.2021 01:40

Spanish, 18.02.2021 01:40

Mathematics, 18.02.2021 01:40


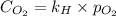
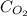 = molar solubility of
= molar solubility of 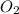 = ?
= ?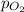 = partial pressure of
= partial pressure of 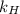 = Henry's law constant = 4.34 × 10⁹ g/L.Pa
= Henry's law constant = 4.34 × 10⁹ g/L.Pa