
For the reaction H2(g) + I2(g) 2HI(g), Kc = 50.2 at 445ºC. If [H2] = [I2] = [HI] = 1.75 10-3 M at 445ºC, which one of these statements is true? A) The system is at equilibrium, thus no concentration changes will occur. B) The concentrations of HI and I2 will increase as the system approaches equilibrium. C) The concentration of HI will increase as the system approaches equilibrium. D) The concentrations of H2 and HI will fall as the system moves toward equilibrium. E) The concentrations of H2 and I2 will increase as the system approaches equilibrium

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2



Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1
You know the right answer?
For the reaction H2(g) + I2(g) 2HI(g), Kc = 50.2 at 445ºC. If [H2] = [I2] = [HI] = 1.75 10-3 M at...
Questions



Mathematics, 19.12.2020 21:30




Advanced Placement (AP), 19.12.2020 21:30


English, 19.12.2020 21:30

English, 19.12.2020 21:30






SAT, 19.12.2020 21:30


Mathematics, 19.12.2020 21:30


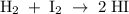
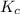
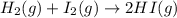
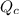 is written as:
is written as:![Q_c=\frac{[HI]^2}{[H_2]^1[I_2]^1}](/tpl/images/0575/8711/8578e.png)
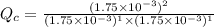
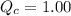
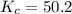
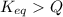 , the reaction will shift towards the right i.e. towards the product side.
, the reaction will shift towards the right i.e. towards the product side.

