
Chemistry, 01.04.2020 19:20 taylor5384
What quantity, in moles, of oxygen is consumed when 717.4 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g)? H2(g) + O2(g) → H2O(l); ΔH° = –285.8 kJ

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:50
Which statement describes how phase changes can be diagrammed as a substance is heated? the phase is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the phase is on the x-axis. the time is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the time is on the x-axis.
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3

Chemistry, 23.06.2019 11:50
How many moles of an ideal gas would occupy a 25.0 liter container when the temperature is 295 k and the pressure is 0.850 atm?
Answers: 2

Chemistry, 23.06.2019 12:40
During an experiment, ice and water were placed in a perfectly insulated thermos flask at 0 °c. describe this system when it phase reaches equilibrium.
Answers: 1
You know the right answer?
What quantity, in moles, of oxygen is consumed when 717.4 kJ of energy is evolved from the combustio...
Questions




Physics, 31.03.2021 01:00


Mathematics, 31.03.2021 01:00

Mathematics, 31.03.2021 01:00

History, 31.03.2021 01:00

Social Studies, 31.03.2021 01:00



Mathematics, 31.03.2021 01:00

Mathematics, 31.03.2021 01:00

Mathematics, 31.03.2021 01:00

Physics, 31.03.2021 01:00





Chemistry, 31.03.2021 01:00

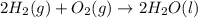
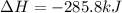
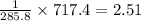 moles
moles

