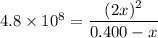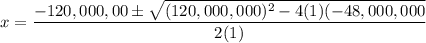H2(g) + Br2(l) ⇄ 2HBr(g) Kc = 4.8 × 108
Assume initial conditions of 0.400 M H2(g) and e...

Chemistry, 01.04.2020 20:16 CHRONICxDJ
H2(g) + Br2(l) ⇄ 2HBr(g) Kc = 4.8 × 108
Assume initial conditions of 0.400 M H2(g) and excess Br2(l). What is the equilibrium concentration of H2(g)?

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3
You know the right answer?
Questions

Mathematics, 05.09.2020 06:01

Mathematics, 05.09.2020 06:01



Biology, 05.09.2020 06:01


Mathematics, 05.09.2020 06:01

Mathematics, 05.09.2020 06:01




Mathematics, 05.09.2020 06:01


History, 05.09.2020 06:01

Mathematics, 05.09.2020 06:01


English, 05.09.2020 06:01

Mathematics, 05.09.2020 06:01


![k_c=\dfrac{[HBr(g)]^2}{[H_2]}=4.8\times 10^8M](/tpl/images/0576/0517/a3b30.png)