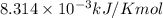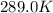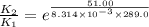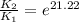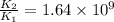Chemistry, 01.04.2020 22:23 milkshakegrande101
The presence of a catalyst provides a reaction pathway in which the activation energy of a reaction is reduced by 51.00 kJ ⋅ mol − 1 51.00 kJ⋅mol−1 . Uncatalyzed: A ⟶ B A⟶B E a = 136.00 kJ ⋅ mol − 1 Ea=136.00 kJ⋅mol−1 Catalyzed: A ⟶ B A⟶B E a = 85.00 k J ⋅ mol − 1 Ea=85.00 kJ⋅mol−1

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1


Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1
You know the right answer?
The presence of a catalyst provides a reaction pathway in which the activation energy of a reaction...
Questions



Mathematics, 22.07.2019 16:00

History, 22.07.2019 16:00

History, 22.07.2019 16:00

Mathematics, 22.07.2019 16:00


Mathematics, 22.07.2019 16:00

Mathematics, 22.07.2019 16:00

English, 22.07.2019 16:00




English, 22.07.2019 16:00


Mathematics, 22.07.2019 16:00

English, 22.07.2019 16:00

History, 22.07.2019 16:00


Health, 22.07.2019 16:00

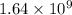
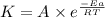
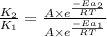
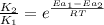
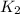 = rate of reaction with catalyst
= rate of reaction with catalyst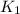 = rate of reaction without catalyst
= rate of reaction without catalyst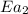 = activation energy with catalyst
= activation energy with catalyst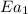 = activation energy without catalyst
= activation energy without catalyst