
Chemistry, 01.04.2020 22:29 ashleyc2442
Given the reaction at equilibrium n2(g) + 3h2(g) ↔ 2nh3(g) increasing the concentration of N2(g) will increase the forward reaction rate due to 1. A decrease in the number of effective collisions

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2
You know the right answer?
Given the reaction at equilibrium n2(g) + 3h2(g) ↔ 2nh3(g) increasing the concentration of N2(g) wil...
Questions

History, 15.11.2019 15:31





Arts, 15.11.2019 15:31

Mathematics, 15.11.2019 15:31

History, 15.11.2019 15:31

Chemistry, 15.11.2019 15:31

History, 15.11.2019 15:31

Computers and Technology, 15.11.2019 15:31



Social Studies, 15.11.2019 15:31


English, 15.11.2019 15:31

Mathematics, 15.11.2019 15:31

Mathematics, 15.11.2019 15:31

English, 15.11.2019 15:31

Biology, 15.11.2019 15:31

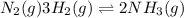 an increase in
an increase in 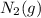 means an increase in the number of reactants.
means an increase in the number of reactants. 

