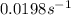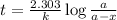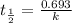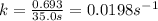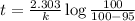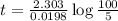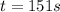Chemistry, 02.04.2020 03:27 cuddleslugsovifat
Be sure to answer all parts. The thermal decomposition of phosphine (PH3) into phosphorus and molecular hydrogen is a first-order reaction: 4PH3(g) → P4(g) + 6H2(g) The half-life of the reaction is 35.0 s at 680°C.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2
You know the right answer?
Be sure to answer all parts. The thermal decomposition of phosphine (PH3) into phosphorus and molecu...
Questions

History, 13.01.2021 14:00


Computers and Technology, 13.01.2021 14:00

Mathematics, 13.01.2021 14:00



Mathematics, 13.01.2021 14:00



Mathematics, 13.01.2021 14:00


English, 13.01.2021 14:00

Mathematics, 13.01.2021 14:00





History, 13.01.2021 14:00

Mathematics, 13.01.2021 14:00

Social Studies, 13.01.2021 14:00

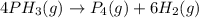 The half-life of the reaction is 35.0 s at 680°C. Calculate the first order rate constant.
The half-life of the reaction is 35.0 s at 680°C. Calculate the first order rate constant.