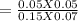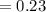An aqueous mixture of phenol and ammonia has initial concentrations of 0.200 M C6H5OH(aq) and 0.120 M NH3(aq). At equilibrium, the C6H5O–(aq) concentration is 0.050 M. Calculate the equilibrium constant, K, for the reaction below.
C6H5OH(aq) + NH3(aq) C6H5O– + NH4+(aq)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:20
Determine which intermolecular forces are the dominant (strongest) forces for a pure sample of each of the following molecules by placing the molecules into the correct bins. drag the appropriate molecular formula to their respective bins.
Answers: 3

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3
You know the right answer?
An aqueous mixture of phenol and ammonia has initial concentrations of 0.200 M C6H5OH(aq) and 0.120...
Questions

Mathematics, 28.01.2021 07:40

Mathematics, 28.01.2021 07:40

Mathematics, 28.01.2021 07:40


Mathematics, 28.01.2021 07:40



English, 28.01.2021 07:40


History, 28.01.2021 07:40

Mathematics, 28.01.2021 07:40




Mathematics, 28.01.2021 07:40



English, 28.01.2021 07:40


![K_{c} =\frac{[C_{6}H_{5}O^{-} ][NH_{4}^{+} ]}{[C_{6}H_{5}OH ][NH_{3} ]}](/tpl/images/0577/3457/a8da2.png)