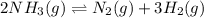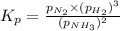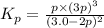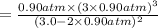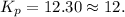Chemistry, 02.04.2020 01:21 leannaadrian
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a 500. ML flask with 3.0 atm of ammonia gas, and when the mixture has come to equilibrium measures the amount of nitrogen gas to be 0.90 atm . Calculate the pressure equilibrium constant for the decomposition of ammonia at the final temperature of the mixture. Round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2


Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1

Chemistry, 23.06.2019 08:00
Why is it important for scientists to review and repeat the work of other scientists? 1.a scientific theory must be tested three times before it is proven. 2.the scientific method only applies to repeated experiments. 3.an experiment may have had errors that the scientists didn't recognize. 4.the results of individual scientists may be influenced by bias. 5.an experiment must be performed twice before the data can be analyzed.
Answers: 3
You know the right answer?
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studyin...
Questions

Mathematics, 20.03.2020 04:21




Mathematics, 20.03.2020 04:22


History, 20.03.2020 04:22





German, 20.03.2020 04:22


Health, 20.03.2020 04:22