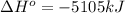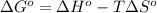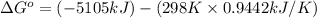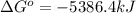Given the values of δhfo in kj/mol and so in j/mol k given below, calculate the value of δgo in kj for the reaction at 298 k: c6h12o6(s) + 6 o2(g) => 6 co2(g) + 6h2o(g) δhfo (c6h12o6) = -1,277 δhfo (co2) = -396 δhfo (h2o) = -242 so (c6h12o6(s)) = 218 so (o2(g)) = 206 so (co2(g)) = 211 so (h2o(g)) = 18

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1
You know the right answer?
Given the values of δhfo in kj/mol and so in j/mol k given below, calculate the value of δgo in kj f...
Questions


Mathematics, 09.03.2021 20:00

Mathematics, 09.03.2021 20:00

English, 09.03.2021 20:00

Mathematics, 09.03.2021 20:00

Mathematics, 09.03.2021 20:00

History, 09.03.2021 20:00

Arts, 09.03.2021 20:00


Mathematics, 09.03.2021 20:00

Mathematics, 09.03.2021 20:00

History, 09.03.2021 20:00

Mathematics, 09.03.2021 20:00





History, 09.03.2021 20:00

Chemistry, 09.03.2021 20:00

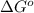 for the reaction is, -5386.4 kJ
for the reaction is, -5386.4 kJ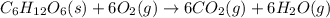
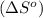 .
.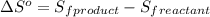
![\Delta S^o=[n_{CO_2(g)}\times \Delta S^0_{(CO_2(g))}+n_{H_2O(g)}\times \Delta S^0_{(H_2O(g))}]-[n_{C_6H_{12}O_6(s)}\times \Delta S^0_{(C_6H_{12}O_6(s))}+n_{O_2(g)}\times \Delta S^0_{(O_2(g))}]](/tpl/images/0577/2061/f660d.png)
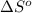 = entropy of reaction = ?
= entropy of reaction = ?![\Delta S^o=[6mole\times (211J/K.mol)+6mole\times (188.7J/K.mol]-[1mole\times (218J/K.mol)+6mole\times (206J/K.mol]](/tpl/images/0577/2061/19137.png)
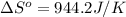
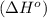 .
.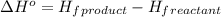
![\Delta H^o=[n_{CO_2(g)}\times \Delta H^0_{(CO_2(g))}+n_{H_2O(g)}\times \Delta H^0_{(H_2O(g))}]-[n_{C_6H_{12}O_6(s)}\times \Delta H^0_{(C_6H_{12}O_6(s))}+n_{O_2(g)}\times \Delta H^0_{(O_2(g))}]](/tpl/images/0577/2061/6b74f.png)
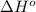 = enthalpy of reaction = ?
= enthalpy of reaction = ?![\Delta H^o=[6mole\times (-396kJ/mol)+6mole\times (-242kJ/mol]-[1mole\times (-1277kJ/mol)+6mole\times (0kJ/mol]](/tpl/images/0577/2061/b27e9.png)