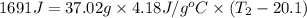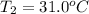Chemistry, 02.04.2020 01:26 kevinkingpin
What is the final temperature of the solution formed when 1.52 g of NaOH is added to 35.5 g of water at 20.1 °C in a calorimeter? NaOH (s) → Na+ (aq) + OH– (aq) ∆H = -44.5 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2
You know the right answer?
What is the final temperature of the solution formed when 1.52 g of NaOH is added to 35.5 g of water...
Questions


Social Studies, 29.08.2019 00:40

Spanish, 29.08.2019 00:50



Health, 29.08.2019 00:50

Mathematics, 29.08.2019 00:50

Mathematics, 29.08.2019 00:50


Mathematics, 29.08.2019 00:50




Mathematics, 29.08.2019 00:50


Mathematics, 29.08.2019 00:50


History, 29.08.2019 00:50


Chemistry, 29.08.2019 00:50

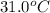
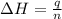
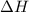 = enthalpy change = -44.5 kJ/mol
= enthalpy change = -44.5 kJ/mol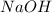 = 1.52 g
= 1.52 g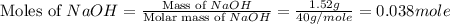
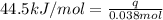
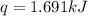
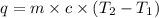
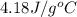
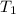 = initial temperature =
= initial temperature = 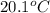
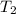 = final temperature = ?
= final temperature = ?