
Chemistry, 02.04.2020 01:42 tyijiapostell
A rigid tank that contains 2 kg of N2 at 25°C and 550 kPa is connected to another rigid tank that contains 4 kg of O2 at 25°C and 150 kPa. The valve connecting the two tanks is opened, and the two gases are allowed to mix. If the final mixture temperature is 25°C, determine the volume of each tank and the final mixture pressure.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
A rigid tank that contains 2 kg of N2 at 25°C and 550 kPa is connected to another rigid tank that co...
Questions

Spanish, 03.02.2021 20:10

Mathematics, 03.02.2021 20:10

History, 03.02.2021 20:10

Biology, 03.02.2021 20:10

History, 03.02.2021 20:10

Mathematics, 03.02.2021 20:10

Mathematics, 03.02.2021 20:10

Mathematics, 03.02.2021 20:10

Biology, 03.02.2021 20:10

Mathematics, 03.02.2021 20:10

Mathematics, 03.02.2021 20:10


Mathematics, 03.02.2021 20:10

Mathematics, 03.02.2021 20:10

Mathematics, 03.02.2021 20:10

Mathematics, 03.02.2021 20:10



Mathematics, 03.02.2021 20:10

Chemistry, 03.02.2021 20:10

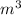
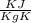
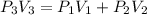 ----- (1)
----- (1)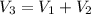
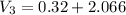
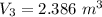
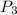 × 2.386 = 550 × 0.32 + 150 × 2.066
× 2.386 = 550 × 0.32 + 150 × 2.066

