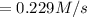Chemistry, 02.04.2020 02:03 recannon02
Consider the reaction: n2(g) 3h2(g) → 2nh3(g) suppose that a particular moment during the reaction, molecular hydrogen is reacting at a rate of 0.0687 m/s. at what rate is molecular nitrogen reacting

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Consider the reaction: n2(g) 3h2(g) → 2nh3(g) suppose that a particular moment during the reaction,...
Questions












Mathematics, 23.05.2020 21:07



Chemistry, 23.05.2020 21:07




Mathematics, 23.05.2020 21:07

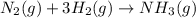
![R=-\frac{1}{1}\frac{d[N_2]}{dt}=-\frac{1}{3}\frac{d[H_2]}{dt}=\frac{1}{2}\frac{d[NH_3]}{dt}](/tpl/images/0576/9841/80d75.png)
![\frac{d[H_2]}{dt}=0.0687 M/s](/tpl/images/0576/9841/7c36a.png)
![\frac{d[N_2]}{dt}=?](/tpl/images/0576/9841/5488c.png)
![-\frac{1}{1}\frac{d[N_2]}{dt}=-\frac{1}{3}\frac{d[H_2]}{dt}](/tpl/images/0576/9841/01276.png)
![\frac{d[N_2]}{dt}=\frac{1}{3}\times 0.0687 M/s](/tpl/images/0576/9841/fd1e5.png)