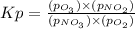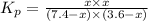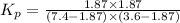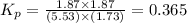Problem PageQuestion Nitrogen forms a surprising number of compounds with oxygen. A number of these, often given the collective symbol (for "nitrogen oxygens") are serious contributors to air pollution. They can often be interconverted, sometimes by reaction with oxygen or ozone () in the air. An atmospheric scientist decides to study the reaction between nitrogen trioxide and oxygen that produces nitrogen dioxide and ozone. She fills a stainless steel reaction chamber with of nitrogen trioxide gas and of oxygen gas and raises the temperature considerably. At equilibrium she measures the mole fraction of ozone to be . Calculate the pressure equilibrium constant for the equilibrium between nitrogen trioxide, oxygen, nitrogen dioxide and ozone at the final temperature of the mixture. Round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2


Chemistry, 23.06.2019 06:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
Problem PageQuestion Nitrogen forms a surprising number of compounds with oxygen. A number of these,...
Questions

History, 16.10.2020 02:01

Mathematics, 16.10.2020 02:01

History, 16.10.2020 02:01

Mathematics, 16.10.2020 02:01


Mathematics, 16.10.2020 02:01

English, 16.10.2020 02:01

Mathematics, 16.10.2020 02:01


Mathematics, 16.10.2020 02:01


Computers and Technology, 16.10.2020 02:01





Spanish, 16.10.2020 02:01

Mathematics, 16.10.2020 02:01


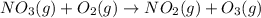
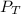 = (7.4-x) + (3.6-x) + x + x = 11 atm
= (7.4-x) + (3.6-x) + x + x = 11 atm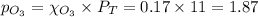
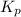 is written as:
is written as: