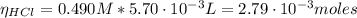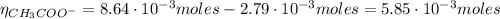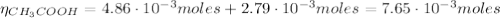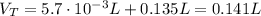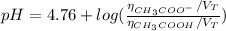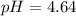Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 04:00
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1
You know the right answer?
A beaker with 135 mL of an acetic acid buffer with a pH of 5.000 is sitting on a benchtop. The total...
Questions


Arts, 02.03.2021 22:40



Mathematics, 02.03.2021 22:40


Mathematics, 02.03.2021 22:40

Mathematics, 02.03.2021 22:40



Mathematics, 02.03.2021 22:40

Mathematics, 02.03.2021 22:40


Advanced Placement (AP), 02.03.2021 22:40

Mathematics, 02.03.2021 22:40


Mathematics, 02.03.2021 22:40

Mathematics, 02.03.2021 22:40


![pH = pKa + log(\frac{[CH_{3}COO^{-}]}{[CH_{3}COOH]})](/tpl/images/0577/1464/a70ac.png)
![5.00 = 4.76 + log(\frac{[CH_{3}COO^{-}]}{[CH_{3}COOH]})](/tpl/images/0577/1464/3fe73.png)
![\frac{[CH_{3}COO^{-}]}{[CH_{3}COOH]} = 1.74](/tpl/images/0577/1464/85deb.png)
![[CH_{3}COO^{-}] = 1.74*[CH_{3}COOH]](/tpl/images/0577/1464/acf42.png) (1)
(1)![[CH_{3}COOH] + [CH_{3}COO^{-}] = 0.100 M](/tpl/images/0577/1464/4ed34.png) (2)
(2)![\eta_{[CH_{3}COOH]} = 0.036 mol/L*0.135 L = 4.86 \cdot 10^{-3} moles](/tpl/images/0577/1464/c8efb.png)
![\eta_{[CH_{3}COO^{-}]} = 0.064 mol/L*0.135 L = 8.64 \cdot 10^{-3} moles](/tpl/images/0577/1464/0b677.png)