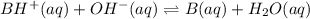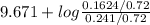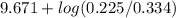Chemistry, 02.04.2020 02:30 dontcareanyonemo
A buffer that contains 0.17 M of a base, B and 0.39 M of its conjugate acid BH+, has a pH of 9.31. What is the pH after 0.02 mol of Ba(OH)2 are added to 0.72 L of the solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2



Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3
You know the right answer?
A buffer that contains 0.17 M of a base, B and 0.39 M of its conjugate acid BH+, has a pH of 9.31. W...
Questions



History, 22.07.2019 17:30


Biology, 22.07.2019 17:30


English, 22.07.2019 17:30


Mathematics, 22.07.2019 17:30


History, 22.07.2019 17:30

Health, 22.07.2019 17:30



History, 22.07.2019 17:30


Mathematics, 22.07.2019 17:30


Mathematics, 22.07.2019 17:30

Mathematics, 22.07.2019 17:30

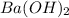 are added to 0.72 L of the solution is 9.5.
are added to 0.72 L of the solution is 9.5.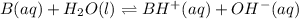
![pK_{a} + log \frac{[B]}{[BH^{+}]}](/tpl/images/0577/1202/d0a56.png)
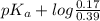
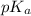 = 9.31 + 0.361
= 9.31 + 0.361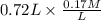
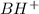 is as follows.
is as follows.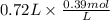
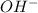 as follows.
as follows.