Chemistry, 02.04.2020 03:13 hmontalvo22
One mole of an ideal gas is isothermally expanded from 5.0 L to 10.0 L at 300 K. Compute the entropy changes for the system, surroundings, and the universe if the process is carried out (a) reversibly by adjusting the pressure of the surroundings to match the internal pressure of the gas, and (b) irreversibly, freely expanding in a vacuum.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
One mole of an ideal gas is isothermally expanded from 5.0 L to 10.0 L at 300 K. Compute the entropy...
Questions


Mathematics, 18.09.2021 03:20

Chemistry, 18.09.2021 03:20





Arts, 18.09.2021 03:20


Mathematics, 18.09.2021 03:20



Social Studies, 18.09.2021 03:20

Mathematics, 18.09.2021 03:20



Mathematics, 18.09.2021 03:20

English, 18.09.2021 03:20

Biology, 18.09.2021 03:20

English, 18.09.2021 03:20

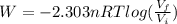



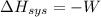


 = -1729 J
= -1729 J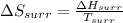
 =
= 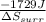


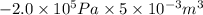
 = 0
= 0 = -W + 1000 J
= -W + 1000 J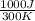
 = -1000 J
= -1000 J