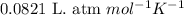Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
A sample of gas at 25°C has a volume of 11 L and exerts a pressure of 0.868 atm. How many moles
Questions

Mathematics, 18.12.2019 03:31



Mathematics, 18.12.2019 03:31


History, 18.12.2019 03:31

Mathematics, 18.12.2019 03:31

Mathematics, 18.12.2019 03:31

History, 18.12.2019 03:31


Social Studies, 18.12.2019 03:31

Mathematics, 18.12.2019 03:31

Computers and Technology, 18.12.2019 03:31






Mathematics, 18.12.2019 03:31

English, 18.12.2019 03:31

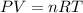
![25^oC=[25+273]K=298K](/tpl/images/0577/4883/df1f6.png)