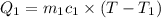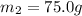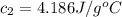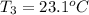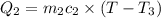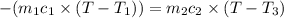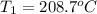Chemistry, 02.04.2020 18:58 makennahudson94
A 62.6-gram piece of heated limestone is placed into 75.0 grams of water at 23.1°C. The limestone and the water come to a final temperature of 51.9°C. The specific heat capacity of water is 4.186 joules/gram degree Celsius, and the specific heat capacity of limestone is 0.921 joules/gram degree Celsius. What was the initial temperature of the limestone? Express your answer to three significant figures.
The initial temperature of the limestone was

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1


Chemistry, 23.06.2019 13:00
Which of the following statements is true about both nuclear fusion and nuclear fission? they occur in the sun. heavy atoms are split. two light nuclei combine. some mass changes into energy.
Answers: 1
You know the right answer?
A 62.6-gram piece of heated limestone is placed into 75.0 grams of water at 23.1°C. The limestone an...
Questions

Mathematics, 21.06.2021 23:40



Mathematics, 21.06.2021 23:40

Mathematics, 21.06.2021 23:40


Mathematics, 21.06.2021 23:40

Business, 21.06.2021 23:40



Computers and Technology, 21.06.2021 23:40



Mathematics, 21.06.2021 23:40






Biology, 21.06.2021 23:40

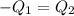
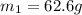
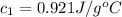
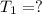
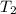 =T = 51.9°C
=T = 51.9°C