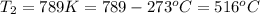Chemistry, 02.04.2020 19:33 jocelynbarraza
A sample of gas in a balloon has a temperature of (+ 23.1oC), a current volume of 15.93 L and a pressure of 763 mmHg. If the balloon rises in the atmosphere and takes on a new volume of 45 .00 L at a pressure of 720 mmHg, what is the temperature at this altitude?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
A sample of gas in a balloon has a temperature of (+ 23.1oC), a current volume of 15.93 L and a pres...
Questions

Advanced Placement (AP), 01.12.2020 08:20

Mathematics, 01.12.2020 08:20



Mathematics, 01.12.2020 08:20

English, 01.12.2020 08:20




Biology, 01.12.2020 08:20

Mathematics, 01.12.2020 08:20


Mathematics, 01.12.2020 08:20


Mathematics, 01.12.2020 08:20

Biology, 01.12.2020 08:20


Mathematics, 01.12.2020 08:20

Advanced Placement (AP), 01.12.2020 08:20

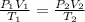
 = initial pressure of gas in balloon = 763 mmHg
= initial pressure of gas in balloon = 763 mmHg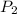 = final pressure of gas in balloon= 720 mmHg
= final pressure of gas in balloon= 720 mmHg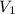 = initial volume of gas in balloon= 15.93 L
= initial volume of gas in balloon= 15.93 L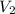 = final volume of gas in balloon= 45.00L
= final volume of gas in balloon= 45.00L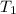 = initial temperature of gas in balloon=
= initial temperature of gas in balloon= 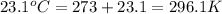
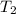 = final temperature of gas in balloon= ?
= final temperature of gas in balloon= ?