
Chemistry, 02.04.2020 22:14 bobbyhill24
A 7.11g sample of potassium chlorate was decomposed according to the following equation: 2KCLO3 -->2KCL + 3O2 How many moles of oxygen are formed?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3
You know the right answer?
A 7.11g sample of potassium chlorate was decomposed according to the following equation: 2KCLO3 --&g...
Questions

Social Studies, 07.08.2019 05:10


Mathematics, 07.08.2019 05:10

Mathematics, 07.08.2019 05:10


Mathematics, 07.08.2019 05:10


Mathematics, 07.08.2019 05:10

Social Studies, 07.08.2019 05:10

Mathematics, 07.08.2019 05:10

Mathematics, 07.08.2019 05:10

Mathematics, 07.08.2019 05:10


Biology, 07.08.2019 05:10


Computers and Technology, 07.08.2019 05:10




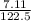
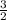 × 0.0580 moles
× 0.0580 moles

