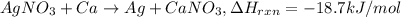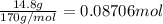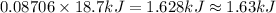Chemistry, 02.04.2020 23:42 ldelgado97
Silver nitrate reacts with calcium to make calcium nitrate and silver. The reaction is exothermic and produces 18.7 kJ/mol of energy. How much energy will be produced when 14.8 g of silver nitrate react. Show your work

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1
You know the right answer?
Silver nitrate reacts with calcium to make calcium nitrate and silver. The reaction is exothermic an...
Questions



Mathematics, 03.03.2020 03:28









Mathematics, 03.03.2020 03:28

Mathematics, 03.03.2020 03:28







Mathematics, 03.03.2020 03:29