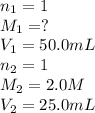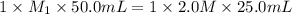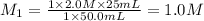BIG POINTS! NO SPAM PLZ! WILL AWARD BRAINLIEST!
Calculate the concentration of hydrochloric ac...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2

Chemistry, 23.06.2019 06:00
What does it mean for something to be dissolved in watera- it is submerged in water moleculesb-it is stirred in the water moleculesc- it is surrounded by water molecules d-it has water molecules added to it
Answers: 2
You know the right answer?
Questions


Spanish, 04.07.2019 00:20

Biology, 04.07.2019 00:20

Mathematics, 04.07.2019 00:20

Mathematics, 04.07.2019 00:20



Computers and Technology, 04.07.2019 00:20

Computers and Technology, 04.07.2019 00:20

Computers and Technology, 04.07.2019 00:20

Computers and Technology, 04.07.2019 00:20

Computers and Technology, 04.07.2019 00:20

Computers and Technology, 04.07.2019 00:20

Mathematics, 04.07.2019 00:20

Biology, 04.07.2019 00:20

Physics, 04.07.2019 00:20

Biology, 04.07.2019 00:20

Social Studies, 04.07.2019 00:20


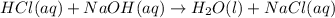
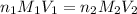
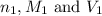 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 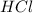
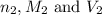 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.