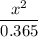Determine the [H3O⁺] in a 0.365 M HClO solution. The Ka of HClO is 2.9 × 10^-8.
Group of...


Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
You know the right answer?
Questions

History, 01.06.2021 18:50


Mathematics, 01.06.2021 18:50

Geography, 01.06.2021 18:50

Mathematics, 01.06.2021 18:50



Social Studies, 01.06.2021 18:50




History, 01.06.2021 18:50

Mathematics, 01.06.2021 18:50

Mathematics, 01.06.2021 18:50




Mathematics, 01.06.2021 18:50


Mathematics, 01.06.2021 18:50

![$ Ka = \frac{[H_{3} O^{+}]\times [ClO^{-} ]}{[HClO]}](/tpl/images/0579/8544/7ce3d.png) = 2.9 ×10⁻⁸
= 2.9 ×10⁻⁸