Chemistry, 03.04.2020 06:06 seannalove6168
A sample of O2 of volume 4.56 L was collected over water at 25c and a total pressure of 1.00 atm. The partial pressure of water is 34.5 Torr. How many moles of O2molescules were collected?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

You know the right answer?
A sample of O2 of volume 4.56 L was collected over water at 25c and a total pressure of 1.00 atm. Th...
Questions

Biology, 11.10.2020 21:01



Mathematics, 11.10.2020 21:01

Mathematics, 11.10.2020 21:01

Spanish, 11.10.2020 21:01



Mathematics, 11.10.2020 21:01


Mathematics, 11.10.2020 21:01


Biology, 11.10.2020 21:01

Business, 11.10.2020 21:01


Biology, 11.10.2020 21:01


Physics, 11.10.2020 21:01

History, 11.10.2020 21:01

Mathematics, 11.10.2020 21:01

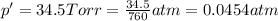
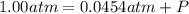
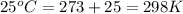
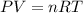 (ideal gas)
(ideal gas)