
Chemistry, 30.08.2019 13:30 gracie0818
After 1.00 ml of water is completely vaporized to gas, how many milliliters of vapor are produced at standard temperature and pressure? (density of water is 1.00 g/ml; molar volume of any gas at stp is 22.4 l/

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2


Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
After 1.00 ml of water is completely vaporized to gas, how many milliliters of vapor are produced at...
Questions

SAT, 29.10.2019 01:31

Social Studies, 29.10.2019 01:31


Mathematics, 29.10.2019 01:31


Chemistry, 29.10.2019 01:31


English, 29.10.2019 01:31

Mathematics, 29.10.2019 01:31

History, 29.10.2019 01:31



Social Studies, 29.10.2019 01:31

Mathematics, 29.10.2019 01:31

History, 29.10.2019 01:31


Mathematics, 29.10.2019 01:31



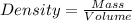
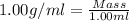
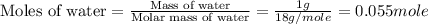
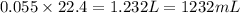 of volume (Conversion factor: 1 L = 1000 mL)
of volume (Conversion factor: 1 L = 1000 mL)


