
Chemistry, 03.04.2020 22:02 wolfiewolffromsketch
Vinegar is a commercial form of acetic acid, HC2H3O2 (aq). One sample vinegar has a pH value of 2.4. State the pH value of a sample that has ten times fewer hydronium ions than an equal volume of a vinegar sample with a pH value of 2.4.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 09:00
The concentration of ionic substances is important for the heart to beat. your heart responds to electrical impulses that travel through heart cells that are made up mostly of water. which properties of ionic compounds are important to support this function? solubility in water conductivity crystalline melting point
Answers: 3

Chemistry, 23.06.2019 10:00
Which number should be placed before f2 on the reactants side equation to make equation balanced? xe + > xef4
Answers: 1
You know the right answer?
Vinegar is a commercial form of acetic acid, HC2H3O2 (aq). One sample vinegar has a pH value of 2.4....
Questions



Mathematics, 24.11.2021 14:00

English, 24.11.2021 14:00

English, 24.11.2021 14:00



Business, 24.11.2021 14:00

Business, 24.11.2021 14:00




Computers and Technology, 24.11.2021 14:00


Mathematics, 24.11.2021 14:00



Biology, 24.11.2021 14:00

English, 24.11.2021 14:00

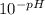
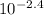
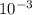 M
M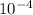 M
M

