
Chemistry, 01.02.2020 09:45 TH3L0N3W0LF
Calculate the freezing point of a 2.6-molal aqueous sucrose solution. the freezing point depression constant for water is 1.86 degrees c/molal.
a.) 4.8 °c
b.) 1.4 °c
c.) -1.4 °c
d.) -4.8 °c

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2

Chemistry, 23.06.2019 06:10
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1
You know the right answer?
Calculate the freezing point of a 2.6-molal aqueous sucrose solution. the freezing point depression...
Questions


Business, 18.10.2019 21:30

Social Studies, 18.10.2019 21:30




Mathematics, 18.10.2019 21:30

Biology, 18.10.2019 21:30


Business, 18.10.2019 21:30



Mathematics, 18.10.2019 21:30







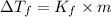
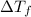 = change in freezing point
= change in freezing point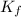 = freezing point constant =
= freezing point constant = 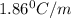
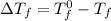
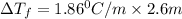
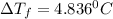
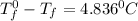
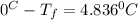
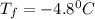
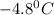 .
.

