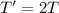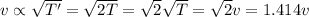Chemistry, 04.04.2020 06:46 brendariobranco
If the absolute temperature of a gas is doubled, what happens to the root‑mean‑square speed of the molecules? Nothing happens to the rms speed. The new rms speed is 4 times the original rms speed. The new rms speed is 2 times the original rms speed. The new rms speed is 1.414 times the original rms speed. The new rms speed is 1/2 the original rms speed.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1

You know the right answer?
If the absolute temperature of a gas is doubled, what happens to the root‑mean‑square speed of the m...
Questions

English, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

English, 18.09.2020 04:01

History, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

English, 18.09.2020 04:01

Social Studies, 18.09.2020 04:01

Social Studies, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

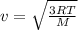
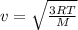
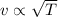 (1)
(1)