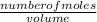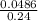Chemistry, 04.04.2020 08:31 Boondiddy9
Suppose that you have 135 mL of a buffer that is 0.360 M in both propanoic acid ( C 2 H 5 COOH ) and its conjugate base ( C 2 H 5 COO − ) . Calculate the maximum volume of 0.240 M HCl that can be added to the buffer before its buffering capacity is lost.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2
You know the right answer?
Suppose that you have 135 mL of a buffer that is 0.360 M in both propanoic acid ( C 2 H 5 COOH ) and...
Questions

Mathematics, 22.12.2019 13:31

Mathematics, 22.12.2019 13:31

Mathematics, 22.12.2019 13:31

Mathematics, 22.12.2019 13:31



English, 22.12.2019 13:31


Advanced Placement (AP), 22.12.2019 13:31

Mathematics, 22.12.2019 13:31

Social Studies, 22.12.2019 13:31

Mathematics, 22.12.2019 13:31

Health, 22.12.2019 13:31



Social Studies, 22.12.2019 13:31

Chemistry, 22.12.2019 13:31

English, 22.12.2019 13:31


Mathematics, 22.12.2019 13:31