
Chemistry, 04.04.2020 10:06 bluebunny1231999
Calculate the pH for the following weak acid. A solution of HCOOH has 0.12M HCOOH at equilibrium. The Ka for HCOOH is 1.8×10−4. What is the pH of this solution at equilibrium?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
Calculate the pH for the following weak acid. A solution of HCOOH has 0.12M HCOOH at equilibrium. Th...
Questions

Mathematics, 11.01.2021 04:40

Mathematics, 11.01.2021 04:40

Mathematics, 11.01.2021 04:40

Social Studies, 11.01.2021 04:40

Biology, 11.01.2021 04:40

English, 11.01.2021 04:40

Mathematics, 11.01.2021 04:40


Mathematics, 11.01.2021 04:40

Biology, 11.01.2021 04:40


Biology, 11.01.2021 04:40




Mathematics, 11.01.2021 04:40

Social Studies, 11.01.2021 04:40

Mathematics, 11.01.2021 04:40

Mathematics, 11.01.2021 04:40

History, 11.01.2021 04:40

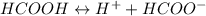
![\Ka= \frac{[H^+][HCOO^-]}{[HCOOH]}](/tpl/images/0582/0861/d7b9e.png)
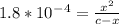
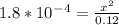
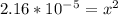
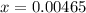
![[H^+] .](/tpl/images/0582/0861/74f2d.png)
![[H^+] = 0.00465 M](/tpl/images/0582/0861/817d9.png)
![pH=-log[H^+]](/tpl/images/0582/0861/15713.png)
![K_{a} = \frac{[H^{+} ][HCOO^{-} ]}{[HCOOH]}](/tpl/images/0582/0861/8c4ca.png)
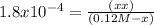
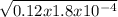 = 4.65 x 10⁻³
= 4.65 x 10⁻³

