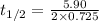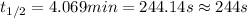Suppose the surface-catalyzed hydrogenation reaction of an unsaturated hydrocarbon has a rate constant of 0.725 M/min. The reaction is observed to follow zero-order kinetics. If the initial concentration of the hydrocarbon is 5.90 M, what is the half-life of the reaction in seconds? *Please report 3 significant figures. Numbers only, no unit. No scientific notation.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2


Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1
You know the right answer?
Suppose the surface-catalyzed hydrogenation reaction of an unsaturated hydrocarbon has a rate consta...
Questions


Mathematics, 24.10.2019 17:43


English, 24.10.2019 17:43


English, 24.10.2019 17:43



History, 24.10.2019 17:43

Mathematics, 24.10.2019 17:43





Mathematics, 24.10.2019 17:43





![t_{1/2}=\frac{[A_o]}{2k}](/tpl/images/0582/1472/b5b11.png)
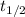 = half-life of the reaction = ?
= half-life of the reaction = ?![[A_o]](/tpl/images/0582/1472/dc622.png) = initial concentration = 5.90 M
= initial concentration = 5.90 M