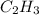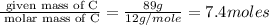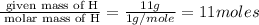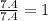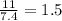Chemistry, 04.04.2020 11:06 lashondrascott
A gaseous hydrocarbon (a compound that contains only hydrogen and carbon) is found to be 11 % hydrogen by mass. a. Find the empirical formula for the compound. A gaseous hydrocarbon (a compound that contains only hydrogen and carbon) is found to be 11 % hydrogen by mass. a. Find the empirical formula for the compound.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3


Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1
You know the right answer?
A gaseous hydrocarbon (a compound that contains only hydrogen and carbon) is found to be 11 % hydrog...
Questions


History, 16.02.2020 13:48

Mathematics, 16.02.2020 13:51

Computers and Technology, 16.02.2020 13:51


Business, 16.02.2020 13:53

Mathematics, 16.02.2020 13:53


Engineering, 16.02.2020 13:53

Mathematics, 16.02.2020 13:55


Engineering, 16.02.2020 14:42

Mathematics, 16.02.2020 14:43

Biology, 16.02.2020 14:44


Medicine, 16.02.2020 14:46

Biology, 16.02.2020 14:46


Mathematics, 16.02.2020 14:47

Mathematics, 16.02.2020 14:47