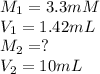Chemistry, 04.04.2020 10:55 hazeleyes2006
Calculate the final concentration of ONPG (in mM) if you add 1.42 mL of 3.3 mM ONPG and dilute to a final volume of 10 mL with PBS buffer. Report your final answer to two places after the decimal.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
Calculate the final concentration of ONPG (in mM) if you add 1.42 mL of 3.3 mM ONPG and dilute to a...
Questions


Mathematics, 08.02.2021 17:50

Mathematics, 08.02.2021 17:50

Business, 08.02.2021 17:50

Mathematics, 08.02.2021 17:50

Mathematics, 08.02.2021 17:50

Spanish, 08.02.2021 17:50



Mathematics, 08.02.2021 17:50

Mathematics, 08.02.2021 17:50

Mathematics, 08.02.2021 17:50

Mathematics, 08.02.2021 17:50

Mathematics, 08.02.2021 17:50





History, 08.02.2021 17:50

Mathematics, 08.02.2021 17:50

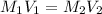
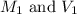 are the initial molarity and volume
are the initial molarity and volume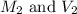 are the final molarity and volume
are the final molarity and volume