
Chemistry, 04.04.2020 11:05 ellaemtagedeane
When iron(III) oxide reacts with aluminum, aluminum oxide and iron are produced. The balanced equation for this reaction is:
Fe2O3 (s) + 2Al (s) > Al2O3 (s) + 2Fe (s)
If 6 moles of aluminum react:
i. The reaction consumes moles of iron(III) oxide.
ii. The reaction produces moles of aluminum oxide and moles of iron.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2


Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
When iron(III) oxide reacts with aluminum, aluminum oxide and iron are produced. The balanced equati...
Questions

Mathematics, 12.03.2021 01:40

Business, 12.03.2021 01:40


Mathematics, 12.03.2021 01:40

Mathematics, 12.03.2021 01:40




History, 12.03.2021 01:40

History, 12.03.2021 01:40

Social Studies, 12.03.2021 01:40






World Languages, 12.03.2021 01:40

Mathematics, 12.03.2021 01:40

Mathematics, 12.03.2021 01:40

Mathematics, 12.03.2021 01:40

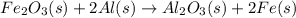
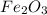
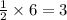 moles of
moles of 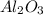
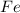
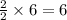 moles of
moles of 

