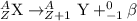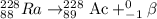Because of the radioactive decay of uranium and thorium in rocks and soil, radium-228, a decay product of Thorium-232, can be found in drinking water. This isotope has a half-life of 5.75 years and an atomic number of 88. If Ra-228 undergoes beta decay, what would the atomic number of the new element be? What would the mass number of this isotope be? Explain your reasoning (e. g. Explain what happens during beta decay).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1


Chemistry, 23.06.2019 09:00
Need ! assume that the variables x and y are directly related. if k = 8, what is the value for each of the following points? be sure and record your data to be used in the following problem. x y k 0.
Answers: 2

Chemistry, 23.06.2019 09:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2
You know the right answer?
Because of the radioactive decay of uranium and thorium in rocks and soil, radium-228, a decay produ...
Questions


Social Studies, 30.10.2019 19:31


English, 30.10.2019 19:31


History, 30.10.2019 19:31

Mathematics, 30.10.2019 19:31






Mathematics, 30.10.2019 19:31





Mathematics, 30.10.2019 19:31

Mathematics, 30.10.2019 19:31